Israeli Group Develops New Electrolysis Technology
By Stephen H. Crolius on October 08, 2019
Last month a group of researchers from the Technion Israel Institute of Technology published a paper, “Decoupled hydrogen and oxygen evolution by a two-step electrochemical–chemical cycle for efficient overall water splitting,” in the journal Nature Energy. The key word in the title is “efficient.” In a September 15 Technion press release, the researchers state that their technology “facilitates an unprecedented energetic efficiency of 98.7% in the production of hydrogen from water.” Applied to the appropriate use case, the technology could lead to a major improvement in green ammonia’s ability to compete with brown ammonia and other low-carbon energy carriers.
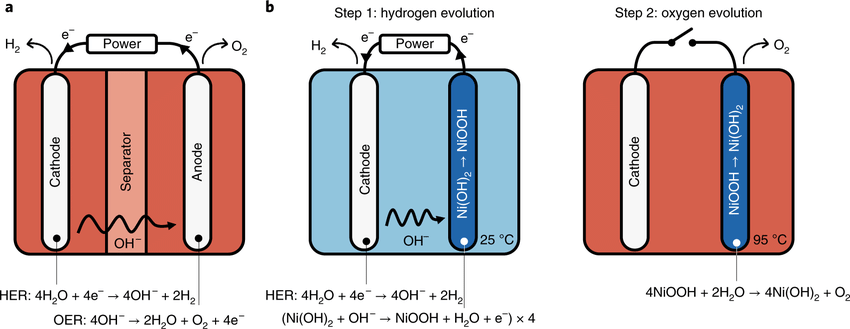
The researchers have named their technology E-TAC, for “electrochemical – thermally activated chemical water splitting.” Whereas conventional electrolyzers use a membrane or diaphragm to physically separate the hydrogen and oxygen generation processes, E-TAC separates the processes in time, via alternating periods of hydrogen and oxygen generation. This regime is carried out with the an architecture that is otherwise the same as a standard electrolyzer, i.e., an anode and a cathode arranged in a liquid electrolyte. When an electrical potential (voltage) is applied to an electrochemical cell of this nature, water is split, with hydrogen ions (H+) migrating to the cathode and hydroxide (OH–) ions migrating to the anode. Upon arrival at the cathode, the hydrogen ions collect electrons that just flowed from the anode and thereby become electrically neutral hydrogen gas (H2). Upon arrival at the anode, the hydroxide ions give up electrons in the process of generating electrically neutral oxygen gas (O2).
The electrochemical magic at the heart of the E-TAC system is based on a property of nickel(II) hydroxide (Ni(OH)2), a compound that is widely used in rechargeable electric batteries. When an anode is composed of Ni(OH)2, the arrival of hydroxide ions during cell operation produces a chemical reaction in which the Ni(OH)2 is converted into nickel oxyhydroxide (NiOOH) plus a quantity of free electrons that can flow to the cathode. This reaction continues until the number of Ni(OH)2 molecules that can be readily converted to NiOOH starts to dwindle. When that point is reached in the E-TAC system, the circuit is interrupted and electric current ceases to flow. In that environment, the NiOOH molecules spontaneously break down into Ni(OH)2 and O2. This process is accelerated by increasing the temperature in the cell. For the bench-scale version, the step 1 and step 2 temperatures were 250 C and 950 C, respectively. When the Ni(OH)2 has been regenerated, circuit continuity is restored and hydrogen generation begins anew.
Electrolysis Context
Electrolysis is seen as a centrally important technology in the sustainable energy economy of the future because it can serve as a bridge between renewably generated electricity and hydrogen-based chemical fuels. With an eye on this opportunity, electrolyzer companies have been striving to expand their focus from the supply of hydrogen for discrete industrial uses to large-scale formats appropriate for the energy sector. For example, the electrolyzer and fuel cell producer Hydrogenics is, per its website, working on “an innovative Power-to-Gas solution [that] integrates renewable sources of generation [and] converts surplus electricity to produce hydrogen or renewable gas . . .” The “energy storage and clean fuel company” ITM Power, per an August 29 press release, will receive funding from the government of the United Kingdom for “deployment of very large scale and hence low cost 100MW+ electrolyser systems using multiple 5MW units; [and] innovations in the siting and operation of these large electrolysers to exploit synergies with large GW scale renewable energy deployments.” According to its website, the industrial conglomerate thyssenkrupp “is currently launching its own large-scale project as part of the cross-sector integration of renewable electricity.” The project integrates a bank of the company’s electrolyzers “with steel mill top gases at thyssenkrupp Steel Europe in Duisburg, Germany, to produce fertilizers and fuel.” And the hydrogen company Nel last month announced a joint project with ammonia producer Yara that will involve integration of a Nel electrolyzer into Yara’s ammonia production process. According to an August 23 Ammonia Energy story, “the partners hope that, by working together to develop Nel’s ‘next generation’ electrolyzer technology and test it in an industrial setting, they can begin to drive down the future cost of renewable hydrogen.” (Hydrogenics, ITM Power, thyssenkrupp, Nel, and Yara are all members of the Ammonia Energy Association.)
Potential Economic Impact
The Technion team contrasts the E-TAC’s 98.7% “energetic efficiency” with a benchmark of “~75% using current methods.” (In their electrolysers brochure, Nel claims that its A Series products comprise “the world’s most energy efficient electrolysers,” with a 79% ratio between the electricity consumed to produce a given quantity of hydrogen and the energy embodied therein.) An advantage of 20 energy-efficiency percentage points sounds big, and it is. It means that a given installation will produce 25% more hydrogen from the same inputs, i.e., the same physical plant and the same amount of purchased electricity. Hydrogen from a 79% efficient electrolyzer whose levelized cost was USD $2.00 per kg would cost USD $1.60 from an E-TAC plant.
Earlier this year, IEA Consultant Julien Armijo and IEA Senior Analyst Cédric Philibert modeled the economics of hydrogen and ammonia production based on solar and wind electricity in advantaged locations in South America. (The paper they submitted to the International Journal of Hydrogen Energy was described in a May 28 Ammonia Energy story.) Hydrogen represents 65% of the cost of ammonia produced in a scenario based on the Tal Tal region of Chile. (The scenario includes an assumption of 70% electrolyzer efficiency.) If electrolyzer efficiency goes up to 98.7%, the levelized cost of ammonia would fall from USD $474 to USD $384 per tonne. At USD $474 per tonne, the Armijo/Philibert green ammonia has a lower cost than the Chilean price of imported ammonia for five of the ten years between 2008 and 2017. At USD $384, the cost is equal to or lower than the average imported price in seven of the ten years, and is meaningfully closer to the prices in the three remaining years.
The Technion team cites its membrane-free architecture as another major advantage for the E-TAC system. The absence of a membrane or diaphragm should allow a reduction in the capital cost of the equipment, perhaps by as much as 50% vs. current electrolyzers. It will also allow E-TAC electrolyzers to function at higher pressures than current electrolyzers in situations where that would facilitate integration with downstream processes.
Commercialization
Sometimes a research breakthrough leads to an impressive technical paper but no near-term move toward commercialization. That is not the case with E-TAC, as is made clear by the Technion press release:
The developers of the technology—Prof. Gideon Grader, Prof. Avner Rothschild and Dr. Hen Dotan—joined together with the founders of the Viber company to establish H2Pro, a company working on commercializing this new technology. Located in the Caesarea Industrial Park, the company was given an exclusive license by the Technion to commercialize the product and to date has raised ~$5 million in a campaign led by Hyundai. H2Pro has more than 20 employees, most of them Technion graduates.
Technion press release, “Fuel of the Future,” September 15, 2019
Gideon Grader’s name will be familiar to members of the ammonia energy community. Grader, a member of the Chemical Engineering faculty at the Technion, oversees a research effort aimed at evaluating the characteristics of nitrogen-based fuels such as urea ammonium nitrate. (This effort was profiled in an Ammonia Energy story that reported on a paper Grader delivered at the 2017 NH3 Fuel Conference.)
Grader confirmed for Ammonia Energy that H2Pro’s projected cost for hydrogen produced by the E-TAC is “considerably lower than alternative electrolysis methods [and] competitive with SMR [steam methane reforming] technology,” while acknowledging “of course, we need to prove these estimates.”
He also confirmed H2Pro’s intention to bring its technology to market on an expedited basis, saying the company intends “to have a commercial unit at the end of 2022.”