New materials for cracking catalysts
By Julian Atchison on September 09, 2021
Among the many challenges for cracking researchers is their choice of material to build their catalysts from. There is hope that cheaper, more readily-available materials will replace the Ruthenium-based catalysts that have dominated the field up to this point. This week two new pieces of research suggest a way forwards using alkali metal-based materials: Lithium and Calcium.
Lithium
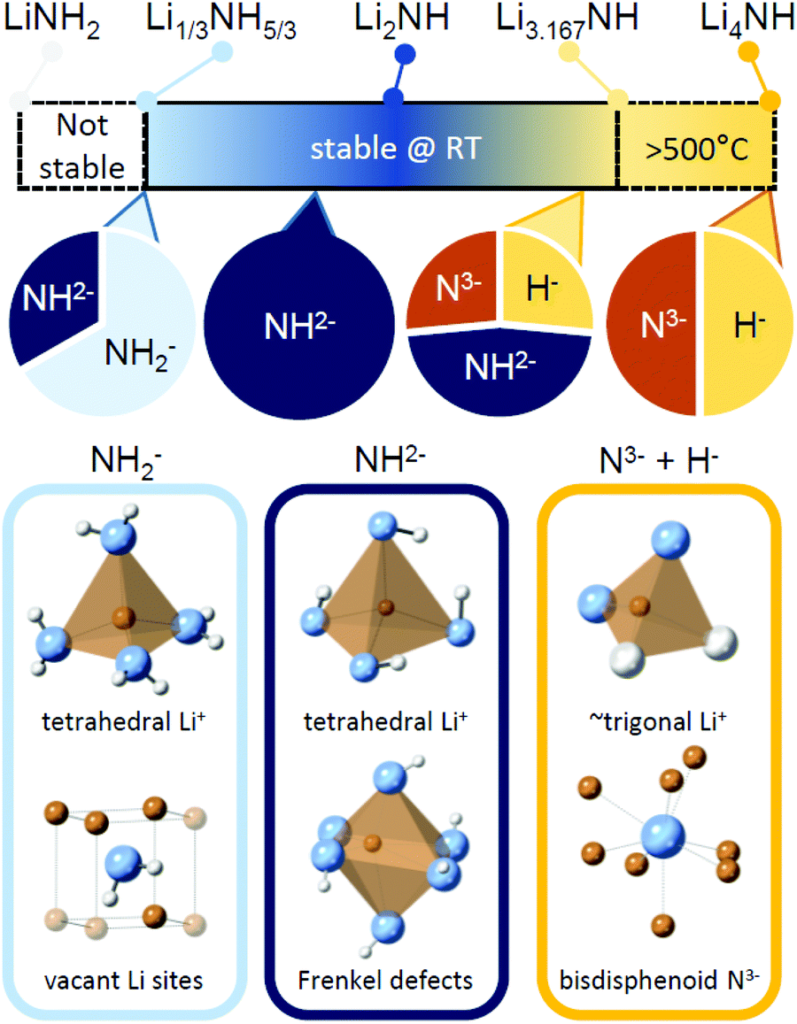
Li-N-H materials are considered a great candidate for hydrogen storage. And, it’s already been demonstrated that the Li–N–H structure can accommodate a wide variety of ions & molecules. But, they may also prove to be highly efficient catalysts for ammonia cracking.
In their recent Physical Chemistry Chemical Physics paper, the Birmingham/Oxford team propose that this structure assists the catalytic process by acting as a “reservoir” for species that might otherwise occupy active sites at the catalytic surface. The team also suggest this is a much-needed evolution in thinking – from seeing catalysts as single-site, “flat” surfaces to multi-site, multi-dimensional structures. This appears to be the best way to “escape the bottleneck” in current research.
Calcium
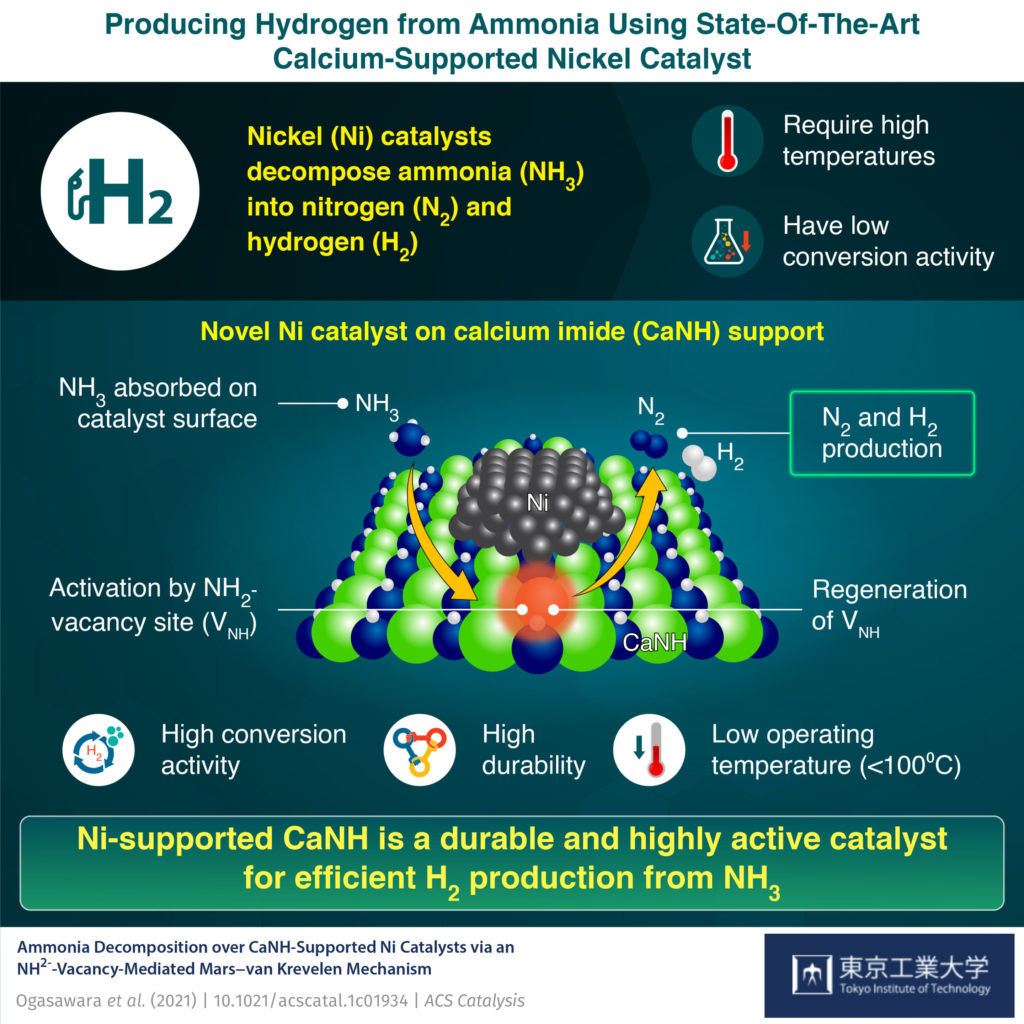
The inclusion of Ca-N-H materials into Nickel-based ammonia cracking catalysts has proven to dramatically increase catalytic activity. In their recent ACS Catalysis paper, the team at Tokyo Tech reported that incorporating Ca-N-H material into the structure results in more vacant, “active” sites at the catalytic surface for hydrogen gas production to occur. They propose this is due to the preferential adsorption of ammonia molecules onto the Ca-N-H structure, and subsequent activation.
Associate Professor Masaaki Kitano was pleased to report the double benefit of his team’s work: not only did they identify a highly efficient ammonia cracking catalyst, but have started to “unravel the elusive working mechanism of such systems”.