New Technology for Generating Hydrogen from Ammonia
By Stephen H. Crolius on March 30, 2017
On March 21, Gifu University in Japan announced a breakthrough in technology for generating hydrogen from ammonia. A press release from the Gifu Prefectural Association Press Club stated that Professor Shinji Kambara, Director of the Next Generation Research Center within the Environmental Energy Systems Department at the Gifu University Graduate School of Engineering, has developed a “plasma membrane reactor” that is capable of evolving hydrogen with a purity of 99.999 percent from an ammonia feedstock. This surpasses the 99.97 percent purity announced last July by a research group centered at Hiroshima University with a hydrogen generation device based on a different technology.
The Gifu technology works at ambient temperature, atmospheric pressure, and does not require a catalyst. The process is characterized by low cost, low environmental impact, and high efficiency, according to Kambara.
Kambara completed development of a first-generation reactor in 2011. This device employed a double-tube structure made of quartz glass, with a ground electrode wrapped around the outer tube and a high-voltage electrode inserted into the inner tube. The tubes were separated by a gap that varied from two to five millimeters. The device produced a non-thermal plasma via the application of voltage with a constant waveform. The plasma in turn, caused gaseous ammonia flowing axially through the plasma field to decompose into diatomic nitrogen and hydrogen (NH3 + e– → 1/2 N2 + 3/2 H2). The device succeeded as a proof of concept but, in this early iteration, fell short of the hydrogen purity needed for real-world applications, such as proton-exchange membrane (PEM) fuel cells.
A plasma consists of a gas in a highly energized state such that electrons dissociate from atomic nuclei, creating ions and radicals (atoms or molecules with an unstable configuration of electrons). While thermal plasmas are generated by elevated temperatures, non-thermal plasmas are generated by strong electromagnetic fields. Non-thermal plasma chemistry has figured in recent advances in ammonia synthesis technology at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and collaborating institutions in Australia; Ondukuz Mayis University in Turkey and Newcastle University in the United Kingdom; and the University of Minnesota in the United States.
In improving his 2011 device, Kambara hit upon the idea of using the hydrogen separation membrane itself as the high-voltage electrode. With the wall of the quartz tube acting as a dielectric barrier (insulator), a “dielectric barrier discharge” generates the non-thermal plasma. As was the case in the earlier version of the device, ammonia molecules passing through the plasma decompose into hydrogen radicals. In the current version, hydrogen radicals are drawn to and through the hydrogen separation membrane of the inner cylinder. This occurs with great specificity, such that other species present in the plasma field fail to pass through the membrane. Once the hydrogen radicals have entered the inner cylinder, they spontaneously pair off to become H2 molecules and exit the device as nearly pure hydrogen gas.
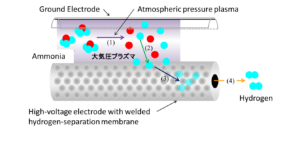
Kambara collaborated with SawaFuji Electric Co., Ltd. in the development of the technology. Sawafuji Electric is a producer of electrical equipment, electronic products, generators, and refrigerators. The company’s work on the hydrogen generation device has led to Japanese patents for a “high-voltage application device” and a “high-voltage application apparatus utilizing pulse voltage and its high-voltage application method.”
The press release states that the range of potential applications for Kambara’s technology is large. “Because liquefied ammonia is easy to transport, it should be more common than liquefied hydrogen and high-pressure hydrogen as fuel for fuel cells.” One important application would be a fuel system for fuel-cell vehicles. “A fuel-cell vehicle is normally filled with 5 kg of hydrogen in a high-pressure tank that occupies 120 liters of space. This [can be replaced by] a 70-liter liquid-ammonia tank and a 50 liter hydrogen-generation unit.”
“From now on,” Kambara is quoted as saying, “hydrogen will be stored as ammonia.”