Content Related to National Renewable Energy Laboratory (NREL)
Industrial load flexibility, the US power grid and ammonia
The Los Angeles Clean Energy Target & ammonia energy
This week the Los Angeles City Council voted to transition to 100% clean energy by 2035, in line with President Biden’s national goals and a decade earlier than the city originally planned. This huge rollout of renewable energy generation is expected to be accompanied by a keystone role for renewable hydrogen, ammonia and synthetic methane for combustion-based power generation.
Ammonia Covered in Forbes.com Power-to-X Review
Last week, Forbes.com published Power-To-X In The German Experience: Another In The List Of Growing Energy Transition Strategies. The article in effect nominates ammonia as a singularly promising up-and-comer in the field of the alternative energy vectors. Such an endorsement is heartening, but the article is notable as much for who is delivering the message – and the fact of its delivery under the Forbes masthead – as for what the message is.
Ammonia: Opportunities for Grid Support
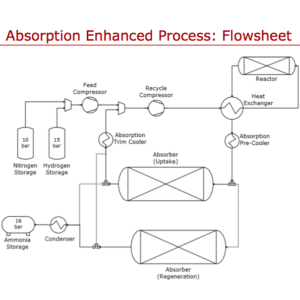
Improvement of Haber-Bosch: Adsorption vs. Absorption
At the recent NH3 Energy+ Topical Conference, Grigorii Soloveichik described the future of ammonia synthesis technologies as a two-way choice: Improvement of Haber-Bosch or Electrochemical Synthesis. Two such Haber-Bosch improvement projects, which received ARPA-E-funding under Soloveichik's program direction, also presented papers at the conference. They each take different approaches to the same problem: how to adapt the high-pressure, high-temperature, constant-state Haber-Bosch process to small-scale, intermittent renewable power inputs. One uses adsorption, the other uses absorption, but both remove ammonia from the synthesis loop, avoiding one of Haber-Bosch's major limiting factors: separation of the product ammonia.
Screening Binary Redox Pairs for Solar Thermochemical Ammonia Synthesis Using Machine Learned Predictions of Gibbs Formation Energies at Finite Temperatures
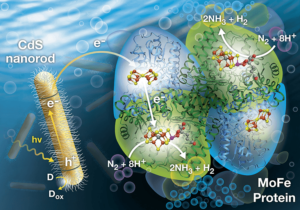
Next-generation ammonia tech: biohybrid nanoparticles
Sustainable ammonia can be produced today: doing so would use electrolyzers to make hydrogen to feed the traditional Haber-Bosch process. In a very few years, new technologies will skip this hydrogen production phase altogether and make ammonia directly from renewable power in an electrochemical cell. Further down the pipeline, next generation technologies will mimic nature, specifically the nitrogenase enzyme, which produces ammonia naturally. One of these next generation technologies is currently producing impressive results at the US Department of Energy's (DOE) National Renewable Energy Laboratory (NREL).
NREL overview of the 2006 conference