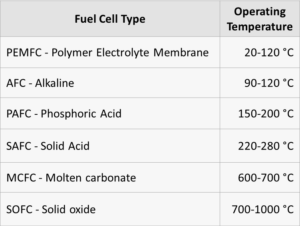
Liquid ammonia can be used as an alternative hydrogen carrier and can be decomposed over catalysts to create a high purity hydrogen stream for fuel cell applications. Ammonia decomposition is typically catalyzed using supported ruthenium catalysts. Current ruthenium catalysts are expensive and often require reaction temperatures of 650 °C to attain complete conversion [1]. For the hydrogen produced from ammonia decomposition to be efficiently used in proton exchange membrane fuel cells, operating temperatures need to be considerably lowered and effluent concentrations of ammonia need to be minimized to avoid poisoning of the membrane [2]. Therefore, it is of interest to…