Catalysts
Technology Status: Anion Exchange Membrane (AEM) Electrolysis
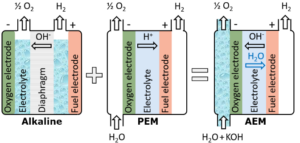
Anion Exchange Membrane (AEM) electrolysis combines concepts from alkaline and PEM. Although AEM can potentially offer the best of both worlds compared to conventional technology, challenges such as oxygen sensitivity, stack scale-up and current density still need to be addressed. Germany-based Enapter is leading the commercialization of AEM systems, with other electrolyzer manufacturers now developing their own products.
PlasmaLeap - Zero Emissions eFuels & Chemicals
Technology status: alkaline electrolysis for renewable ammonia production
Alkaline electrolyzers will play a significant role in renewable ammonia production going forward. Historical developments in electrocatalysts and optimized stack design have already addressed some of the key bottlenecks in the technology, and new developments will enable flexible operations at higher pressures.