Production technology updates: from mega-scale to distributed ammonia
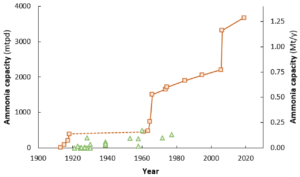
Recently, KBR launched its Ammonia 10,000 technology for newbuild ammonia plants, tripling the largest available single train capacity to 10,000 metric tonnes per day. In our latest Technology Insights article, we explore the other pieces of the puzzle required for mega-scale ammonia, as well as some updates from the other end of the spectrum, with three distributed, small-scale ammonia synthesis systems under development in North America.