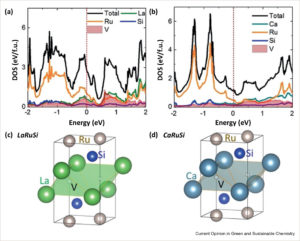
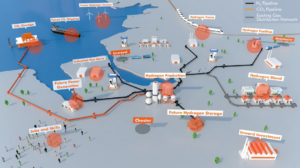
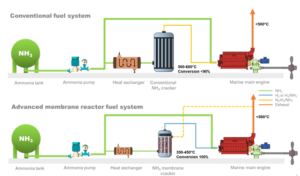
DECHEMA and Fertilizers Europe: decarbonizing ammonia production up to 2030
DECHEMA and Fertilizers Europe recently released a new report detailing how & where the European fertilizer industry can decarbonize leading up to 2030. Technology options for CO2-emission reduction of hydrogen feedstock in ammonia production explores decarbonization pathways including energy efficiency improvements, carbon capture & sequestration, renewable hydrogen feedstock and grid-based electrolysis. It proposes a detailed roadmap towards 19% emissions reduction from the EU fertilizer industry by 2030, and – looking ahead to 2050 – forecasts the almost complete decarbonization of the industry, via zero-carbon electricity generation in the EU and the growth of renewable hydrogen production. With the right policy & regulatory levers in place, Fertilizers Europe believes there is no reason the transition cannot happen faster.